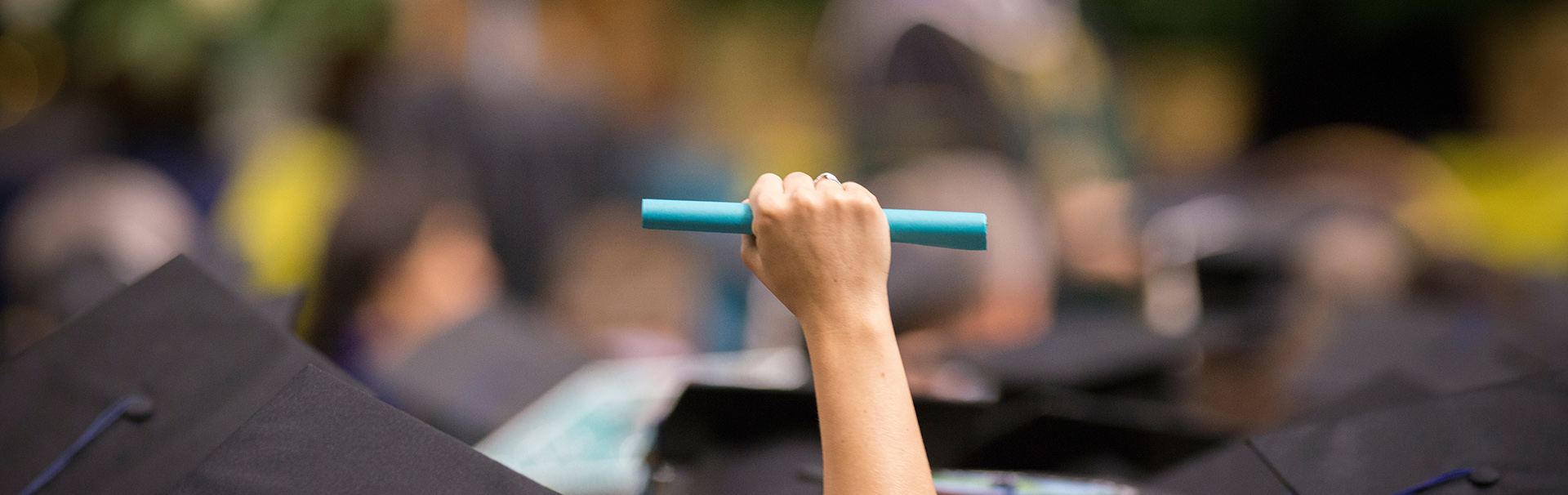Get the Career Service You Need
See How We Help
Contact Information
Career Center
Phone: 910.962.3174
Fax: 910.962.4257
601 S. College Rd.
Fisher University Union 2035
Wilmington, NC 28403-5924
Fall & Spring Office Hours:
Monday - Friday: 8 a.m. - 5 p.m.
Express Hours: Fall & Spring Semesters
Monday - Friday: 1 p.m. - 4 p.m.